Prediction medical device for rheumatoid arthritis
PREDIRA will validate a revolutionary online platform that analyses blood samples to let doctors determine how well various biotherapies for rheumatoid arthritis will work for each individual patient. Based on biomarkers, the test can prevent a patient from spending months on the wrong treatment.
Origins
Rheumatoid arthritis, a degenerative disease, involves chronic disabling pain, inflammation and stiffness. It is the most common inflammatory systemic autoimmune disease, affecting 33.4 million people worldwide. It typically affects people over 40 and the consequences are expected to increase over the next 10 years in Europe. PREDIRA validates an innovative solution to improve rheumatoid arthritis treatment by ranking the efficiency of biotherapies for each patient.
Team
- SERMAS integrates every public hospital and public health service of the Madrid Regional Public Health System.
- IESE: The Center for Research in Healthcare Innovation Management (CRHIM) at IESE implements excellent research in innovation management in healthcare.
- UGA- GREPI is a laboratory specialised in discovery of biomarkers of interest in rheumatology.
- SINNOVIAL: Medtech Company develops innovative personalised-medicine devices for chronic inflammatory rheumatism management.
The project
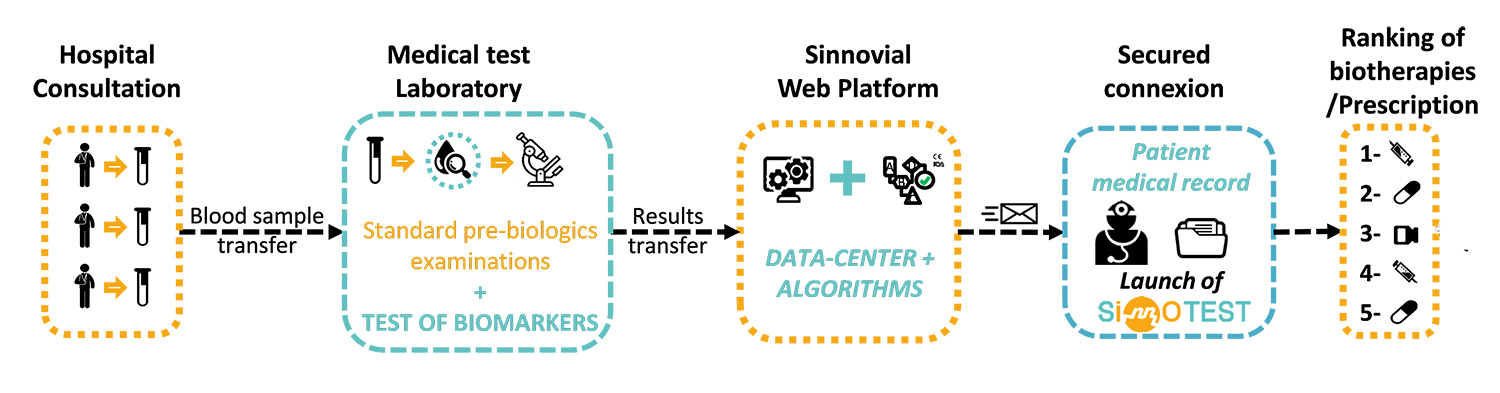
SinnoTest® is a medical device allowing ranking of the efficacy of biotherapies for rheumatoid arthritis. From a simple blood sample, SinnoTest® is able to determine the likelihood of biotherapy treatment response, based on analysis of the patient’s specific protein biomarkers. SinnoTest® thus enables optimal biotherapy selection for 90% of all patients. The answer is given immediately, so no time is lost in unsuccessful trials of therapies that won’t work. This tool will disrupt the whole chain of treatment prescription in the rheumatology field. Because it uses classical, validated and routine biological analysis techniques, SinnoTest® is easily accessible to all practitioners in rheumatology in Europe and worldwide.
Robust and innovative proprietary algorithms for three biotherapies (Infliximab, Etanercept and Adalimumab) were generated, clinically validated and implemented in the first online SinnoTest® software version. This system will be used by hospital clinicians in 2019 as an investigational device in a feasibility assessment financed by SINNOVIAL. Three more algorithms will be clinically validated during the PREDIRA project.
The objective of the PREDIRA project is to confirm the performance of SinnoTest® on a new cohort of 200 patients in Spain, to facilitate distribution of this innovative predictive test throughout Europe as soon as possible. The project will also analyse European health systems in order to better integrate SinnoTest® into the patient care path.
Impact
If rheumatologists can immediately choose the right biotherapy for individual patients, it means improved quality of life for patients needing treatments, because no precious time is lost in unsuccessful biotherapy trial and fails, which can cause up to 18 months of wasted time. It also means big savings for payers, as unsuccessful biotherapies can cost €7 billion for the whole EU healthcare system.
Why this is an EIT Health project
This project is in keeping with the EIT Health Focus Area of “Improving Care Pathways”, because it promises faster, more accurate diagnoses that allow for personalised therapies. It is also in keeping with the overall EIT Health mission to improve healthcare for European citizens.
External Partners
Partners

CLC/InnoStars: Spain
Partner classification: Business, Education
Partner type: Associate Partner
IESE Business School is dedicated to training professional managers, with international educational programmes, including those with a particular focus on innovation management in the Health sector. IESE Centre for Research in Healthcare Innovation Management (CRHIM), created in 2012, builds on over 30 years of expertise in the sector. It enables public and private R&I projects to become a reference centre for health innovation management. Specialist areas include: Health management, health economics, business development, executive education, capacity-building activities.
IESE Business School (CHRIM)
IESE Business School (CHRIM), Av. de Pearson, 21, 08034 Barcelona, Spain
Key Activities in Business Creation
Finance & Investment, Business coaching
Key Activities in Education
Business Schools, Entrepreneurship training, Technical faculties, Healthcare professional education/training






CLC/InnoStars: Spain
Partner classification: Municipality / City, Hospital / University Hospital
Partner type: Core Partner
Servicio Madrileño de Salud (SERMAS) is the public health provider of the region of Madrid. SERMAS belongs to the Spanish National Health System and provides services to more than 6 million citizens through 38 hospitals and 424 primary care centres. SERMAS is an international reference for high-specialized medicine; it is equipped with state-of-the art stage technologies and characterized by high-qualified health professionals distributed in three domains: primary care, hospital care and emergency care through SUMMA 112. SERMAS has one of the best public primary care systems in good coordination with hospital care and social services in order to provide integrated care and achieve real impact on patients and families. In order to improve health research management and coordination, SERMAS works with 13 Research Foundations that support from the economic and administrative point of view research and innovation that originates at university hospitals, primary care, the emergency medical service and public health covering all areas of specialties and including communication and information technologic departments. These public research foundations focus on innovation and translational research, seeking for real outcomes in healthcare. SERMAS is committed to ensure the continuous improvement of quality.
Key Activities in Social Innovation
Healthcare provision, Payers
Key Activities in Business Creation
Technology Transfer, Testing & Validation
Key Activities in Education
Medical faculties, Healthcare professional education/training






CLC/InnoStars: France
Partner classification: Education, Research, Tech Transfer, Clusters, Other NGOs
Partner type: Core Partner
The UGA in Grenoble is a leading University of Science Technology and Health. Within 80 multidisciplinary laboratories, the UGA is developing outstanding research at national and international level. The UGA offers a wide range of training courses, from bachelor to doctorate, in close connection with the socio-professional environment to promote the integration of its students.
Université Grenoble Alpes (UGA)
621 Avenue Centrale, 38400 Saint-Martin-d'Hères
Key Activities in Research and Developement
Biomedical engineering, Life science, Social sciences /health economics
Key Activities in Corporate Innovation
Pharma, Med Tech, ICT, Diagnostics, Imaging, Nutricion
Key Activities in Business Creation
Incubation, Technology Transfer, Business coaching
Key Activities in Education
Entrepreneurship training, Technical faculties, Medical faculties, Healthcare professional education/training


