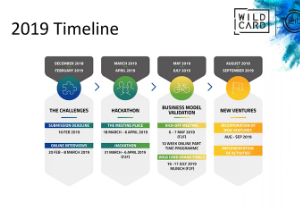
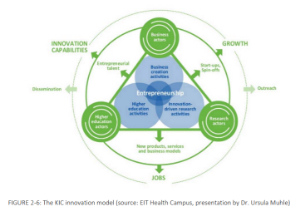
Read the latest news updates from across our organisation.
Health experts make recommendations on EHDS implementation

Discover our new Think Tank report.
EIT Health-supported ABLE Human Motion receives CE mark

Learn how EIT Health has supported their…
EDIT-B launches the first blood test that can differentiate bipolar disorder from depression in France

Hear from those diagnosed with Bipolar Disorder
María González Manso wins 2024 European Prize for Women Innovators

Plus, three EIT Health-supported innovators recognised as…
Over €500M raised by EIT Health Gold Track alumni

45 companies supported through the programme.
€37M raised by Women Entrepreneurship Bootcamp alumni

Learn how we support women-led start-ups.
EIT Health joins “Seeds of Bravery” Consortium

Discover how we will support Ukrainian start-ups.
EIT Health renews strategic alliance with HIMSS Partner Innovation Exchange (PIE) Initiative

Read more about the partnership
20 scale-ups selected for EIT Health Bridgehead 2023

20 healthcare scale-ups receive customised assistance
ProHerz gains DiGA status following EIT Health support

ProHerz recognised as reimbursable digital health app
Catapult 2023 Winners Announced at Bits & Pretzels

deepeye, immunyx, and timeisbrain win EIT Health…
Nia Health closes €3.5M seed financing round

Expanding AI and development capabilities
EIT Health named top business accelerator by UBI Global

Learn more about the achievement
EIT Health-supported S4DX closes €3.6M financing round

Learn more about the Wild Card alumni
EIT Health and IHI unite to drive healthcare innovation

Read about our joint commitment
European Digital HealthTech Hub Conference 2023

Supporting DMDs from incubation to market
EIT Health project EDIT-B launches new test for bipolar

Celebrate World Bipolar Day with us
MiMARK shifts cancer care model with EIT Health support
Highlighting importance of developing new models to…
Four more EIT Health-supported start-ups gain EIC grants

Learn more about their achievements.
EIT Health set to bridge innovation gap in Austria

We have launched a new Co-Location Centre…
Three EIT Health Catapult alumni receive EIC grants

Learn more about the start-ups that received…
NALA.care joins forces with La Roche-Posay

Discover how the partnership will benefit patients
EIT Health appoints three new Supervisory Board members

Join us in welcoming our new Board…
Crises spur need to bridge the innovation gap in Europe

Learn more about INNOVEIT Paris and Warsaw
EIT Health drives digitalisation in cardiovascular care

Learn more about CVD solutions
Pandemic underlines need for “One Health” approach

Learn about the INNOVEIT Paris key theme
EIT to support EIT Health for a further seven years

EIT have confirmed the partnership
InCephalo AG receives EIT Health Gold Track grant

Expanding its compartment-locked technology
Estonian healthtech start-up Antegenes raises €2.3M

Funding will be used to broaden the…
WildCard winner iLoF secures further $5M

Funds to accelerate development of treatments
Ganymed Robotics raises €21M for global expansion

Ganymed Robotics took part in Bridgehead Global
EIT Health-supported PIPRA concludes €2.1M seed round

Learn more about this recent success.
EIT Health-supported Diabeloop raises €70M

Learn more about Diabeloop's successful funding
Leveraging the innovative power of the healthtech system

Learnings from Bits & Pretzels HealthTech
Annika Szabo Portela appointed MD EIT Health Scandinavia

Meet EIT Health Scandinavia's new MD
42 start-ups through to Catapult 2022-23 semifinals

Discover the promising start-ups chosen
Pandemic spurs move to increased patient centricity: Is it here to stay?

Learnings from EIT Health Summit 2022
EIT Health Catapult 2021-22 names winners

Ebenbuild, Leuko and SolasCure crowned champions
EIT Health-supported IMPLICITY raises €21M

Funding will help international expansion and fuel…
First annual Bits & Pretzels HealthTech announced

EIT Health and Roche are founding partners
EIT Health-supported Optellum attains CE mark
Innovative lung cancer diagnosis solution
Call for tech giants to back talent crunch initiative

The WorkInHealth Foundation seeks donors
Institut Mérieux joins Venture Centre of Excellence

Empowering finance for EU health SMEs
Jean Marc Bourez appointed interim CEO of EIT Health

Jean Marc Bourez has been appointed CEO…
Healthy Mind raises €1M to help patients with anxiety

EIT Health supported them towards investment
Orthox closes $12.5M in Series A funding

Investment will support further clinical trials of…
CroíValve raises €8M in Series A funding round
Investment will support feasibility study
New event partnership advances High Value Care agenda

Redefining Health Care Summit 2022
Platform shows strong results for diabetes prevention

Health Integrator is backed by EIT Health
EIT Health joins call to ‘Close the Care Gap’ on cancer

Supporting early screening and diagnosis
1,750 professionals ready to embrace AI in healthcare

EIT Health launches HelloAI RIS and HelloAI…
EIT Health partners unite to advance lung cancer diagnosis

GE Healthcare and Optellum join forces
EHDS report connects innovators and European Commission

Report supports European Commission's EHDS creation
24 start-ups selected for Bridgehead programme

Support to expand presence to new markets
First partner meeting ahead of Austrian hub launch

Partners meet ahead of January 2022 opening
EIT Health and Biogen launch ‘neurotechprize’

Addressing Alzheimer’s Disease from around the globe
EIT Health and Novartis launch `Beyond Rheum´

Supporting patients with Axial Spondyloarthritis
EIT Health-supported PhagoMed acquired by BioNTech

Accelerated under our Gold Track programme
EIT Health wins at the 2021 Association Excellence Awards

The ‘Making Connections’ platform wins silver
EIT Health announces new Supervisory Board

Including new Chairwoman, Lisa Shaw-Marotto
21 start-ups selected to innovate for healthy ageing

21 start-ups have received a Catalyst Award
EIT Health RIS triumphs at the Emerging Europe Awards

Winning best Regional Collaboration Initiative 2021
EIT Health’s Bridgehead first programme with EIC

To scale European start-ups and drive innovation
EIT Health-supported Optellum partners with J&J

Aiming to increase lung cancer survival rates
EIT Health to welcome 6 new partners in live event

Strengthening its network of healthcare innovators
EIT Health start-up partners with Astellas

Researching therapies for mitochondrial dysfunction
EIT Health RIS Innovation Call 2021 supports 11 new ideas

Up to €75 000 for proof-of-concept projects
EIT Health project wins ‘social bond of the year’

Addressing the burden of chronic disease
EIT Health & EIC partner to support start-ups

Providing access to networks and financial opportunities
EIT Health start-up raises EUR 1m in seed funding

Longenesis receive investment to digitise biomedical research
EIT Health Catapult 2021 selects 42 semi-finalists

Learn about these promising start-ups
EIT Health partners in Warsaw Health Innovation Hub

A joint initiative between government and business
EIT Health’s S4DX raises €5M to digitise blood sampling
Successful funding round for Wild Card winner
EIT Health supported Sleepiz AG secures EUROSTAR grant

To help sufferers of sleep apnea
EIT Health Bootcamp selects 11 female-led start-ups

Connecting women-led teams to mentors and investors
EIT Health selecting three Supervisory Board members

Independent members sought; apply by 23 May
Bridgehead announces first start-ups for 2021

Helping SMEs expand in Europe and beyond
EIT Health rewards societal impact at Pitch Contest

Amparo takes 1st place at MedtecLIVE
EIT Health supported Optellum in AI world-first

Breakthrough in lung cancer detection technology
EIT Health-entrepreneur on Medicine’s Maker ‘Power List’

Evelina Vågesjö, CEO at Ilya Pharma
EIT Health launch new report on AI in health

Urging healthcare providers to embrace AI and…
EIT Health-supported start-up raises $10m
Oxford Endovascular's successful funding round
Peptomyc start human trials of ‘universal cancer therapy’

First-in-human trial approved for novel Myc inhibitor
EIT Health and BioMed Alliance join forces

Signing Memorandum of Understanding (MoU) for two…
EIT to boost innovation in higher education

New initiative to unlock potential of HEIs
Italian Allelica raises $1.75M and goes international

International success of Italian start-up Allelica
Obituary: Jaime Prat Pastor, EIT Health Spain

General Secretary, EIT Health Spain Governing Board
Renewed partnership for HealthTech Innovation Days

Promoting access to healthcare for all
Four exciting companies receive expert mentoring

EIT Health helps promising firms commercialise
EIT Health supported start-up Unhindr awarded £500,000

Unhindr has won funding to scale AI…
EIT Health appoints two new Supervisory Board members

Dr Angela Spatharou and Felix Faucon
Venture Centre of Excellence launches call for VCs to join

Partnership with EIT Health will finance start-ups
EIT Health expands to Finland as HUS becomes partner

The country's largest healthcare provider
EIT Health Catapult names 2020 winners

The most promising European healthcare start-ups
Christina Petris new EIT Health Ireland & UK Managing Director

New Managing Director for EIT Health Ireland…
Second start-up launch on EIT Health crowdfunding site
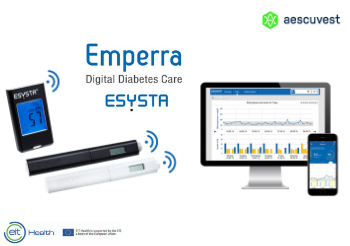
Emperra launches on EIT Health crowdfunding platform
EIT Health Catapult winner raises €44M for gene therapy

SparingVision on track for first human trials
21 start-ups reach EIT Health Catapult Final

Among Europe's leaders in biotech, medtech, digital…
Four EIT Health innovators in running for EIT Awards

28 of Europe’s top innovators will compete
EIT Health names winning longevity innovations

21 Catalyst Awards administered by Headstart
Six visionary start-ups selected for Gold Track

EIT Health helps promising firms go global
Headstart winner Sleepiz earns CE certification

EIT Health-backed start-up deals in sleep monitoring
EIT Health celebrates UN’s Day of Older Persons

Event puts focus on healthy and active…
EIT Health project wins Horizon Impact Award
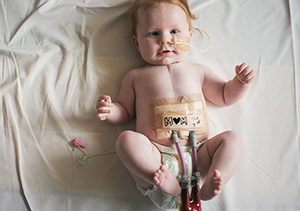
MACH supports children awaiting transplants
Registration opens for EIT Health Summit Series

Sessions run from 24 November-3 December 2020
Dr. Volker Lodwig joins EIT Health Supervisory Board

He will represent EIT Health Germany’s Partners
Oncology firm accelerated by EIT Health receives €1M
Headstart winner SideROS raises investment for cancer…
EIT Health Summit Series will run online 24 Nov-3 Dec

Public are welcome to our biggest annual…
AI tool for COVID-19 produces significant results
EIT Health project aids personalised medicine
EIT Health helps Tubulis secure €10.7M in funding

Investor Network aids firm doing antibody work
EIT Health-backed start-ups garner big investments

A million each for iLoF and Restorative…
EIT Health aids 11 start-ups disrupted by COVID-19

Start-up Rescue Instrument gives €5.5M in co-investment
2020 Think Tank topic: AI and healthcare transformation
‘Workforce and Organisational Transformation with AI –…
EIT Health CEO speaks on data and health at conference

‘In five years’ time, there won’t be…
Virtual Wild Card Hackathon kicks off

Seeking solutions for women’s health and digital…
EIT Health drives plan to pay for health, not illness

Former Innovation Project develops health bond scheme
EIT Health RIS Innovation Call backs 16 healthcare consortia

Innovations from eight countries in emerging Europe
42 start-ups reach EIT Health Catapult Semi-finals

Read more about the future game changers
Bridgehead Europe helps 15 more start-ups go abroad

Meet the firms expanding in Europe
European Youth Parliament partners with EIT Health

EYP brings together young Europeans to address…
Professor Gregory Katz on value-based healthcare

Q&A covers EIT Health's recent publication
Paris partnership benefits Venture Centre of Excellence

HealthTech For Care and EIT Health collaborate…
EIT Health submits report to EC on strategy for AI and data

Consultative group formed to contribute to discussions
Think Tank report: ‘Optimising the Innovation Pathway’

Recommendations from 2019 Round Tables
EIT Health selects start-ups for Headstart 2020

96 start-ups receiving mentoring and financial support
EIT Health launches guidance on Value-Based Health Care

To improve patient outcomes and reduce waste
Ten start-ups receive Living Labs and Test Beds support

€7,500 vouchers cover EIT Health Accelerator programme
Bridgehead Global to help 21 firms expand beyond Europe

EIT Health programme winners announced 6 May
EIT Health start-up aid part of EIT’s COVID-19 response

Start-ups can apply for co-investment
EIT Health’s crowdfunding effort helps fight COVID-19
#CrowdBeatsCorona – Aescuvest helps start-ups, research firms
EIT Health pledges close to €7M for COVID-19 solutions

Fifteen "Rapid Response" projects are funded
Bridgehead to aid 15 start-ups in European expansion

EIT Health programme names 1st round of…
Céline Carrera is new EIT Health Education Director

She will develop the education strategy and…
Founders of Wild Card start-up join Forbes 30 Under 30

iLoF facilitates Alzheimer's testing.
EIT Health, McKinsey & Co. on AI: invest in professionals

New report on how AI impacts healthcare.
Retour sur la 5ème édition de Neuroplanète, soutenue par EIT Health France

La 5e édition s’est tenue les 6…
Bart Haex is EIT Health Director of Strategy (ad interim)

Bart will lead the process of further…
#ProtectUrLife helps head off health risks

Effort screens thousands, raises awareness of heart,…
EIT Health establishes new office in Denmark

Copenhagen office expands the network’s reach
Investor Network holds pitch contest at EIT Health Summit

Fondation de l’Avenir and Matmut sponsor contest
20 start-ups funded in second round of Headstart

EIT Health Belgium-Netherlands and EIT Health Germany…
Headstart Day in France: Extra funding, mentoring

EIT Health France gives extra support to…
EIT Health coordinates launch of Hub in Tel Aviv

European, Israeli innovation ecosystems linked.
EIT Health France aids start-ups in Network Call pilot

EIT Health France Network Call pilot programme…
FREDMILL with the first prize of the EIT Health StarShip 2019

6 November 2019, in Lisbon during an…
Forty students graduate from EIT Health Master’s programmes
Next generation of health innovators attends EIT…
European Health Catapult winners announced

The biggest pitch contest organised by EIT…
EIT Health Summit 2019 focuses on the patient

More than 1 000 delegates meet in Paris.
Italian pharmaceutical company association interested in EIT Health opportunities

Italian partners of EIT Health met Farmindustria…
InnoStars Talks – how could personal experience successfully revolutionise the healthcare market?

InnoStars Talks is a series of interviews…
Restorative Neurotechnologies wins the 2019 EIT Health InnoStars Headstart 2019

Restorative Neurotechnologies, a spin-off from
Two new EIT Health Wild Card start-ups begin their journey with up to €2 million in funding

Addressing the challenge of Mental and Brain…
EIT Health Degree Programmes on ageing brains and data analytics earn prestigious EIT Label

They join four other EIT Health education…
EIT Health-backed initiative on data in healthcare is launched at the European Patients’ Forum Congress

Raising awareness about responsible use of health…
EIT Health Accelerator programme receives global recognition of impact and performance

UBI Global also recognises EIT Health Partners
World Stroke Day: EIT Health Innovation Projects facilitate rehab
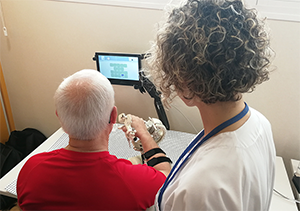
Some EIT Health projects facilitate stroke rehabilitation…
EIT Health holds panel on AI and ethics at the World Health Summit

EIT Health presents its AI report on…
EIT Health invites start-ups to join global competition targeting improvements in healthy longevity

More than $30 million in prizes to…
EIT Health-backed innovation for children with type 1 diabetes triumphs in prestigious European awards

Marc Julien of Diabeloop wins EIT Innovators…
EIF and EIT Health collaborate for Europe’s health

Memorandum of Understanding signed 13 September 2019
EIT Health Investor Network helps French start-up raise €1.8m to combat loneliness in chronic disease
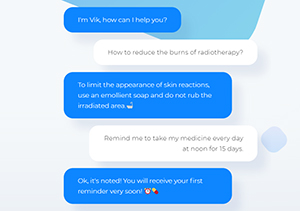
Wefight’s virtual companion "Vik" to be made…
Innovative Campus summer school makes physical education inclusive

InPhysEd is all set to run in…
Report notes EIT Health’s role in Munich Life Sciences ecosystem

EIT Health management and partners interviewed
EIT Health partners with UBI Global innovation intelligence company and community

UBI Global has a network of over…
Peptomyc publication details preclinical validation of new inhibitor to treat cancer

EIT Health-supported team fights skepticism in its…
EIT Health France’s Plaza Open Day

Assistance provided for projects submission on Plaza
EIT opens Silicon Valley Hub, boosting EIT Health access to US market
Transatlantic bridge for European innovation
Wild-Card Hackathon: Top entrepreneurs compete to tackle health challenges

30 competitors form teams and begin the…
EIT Health Alumni share experiences of Industry & Innovation Day

Event held 1-3 March 2019 in Mannheim,…
Dr. Cristina Bescos is new Managing Director of EIT Health Spain

She replaces Dr. Marco Pugliese, who shepherded…
SelfDiagnostics from Estonia presents at MedTech Strategist Forum for investors

Joining French SME Aenitis as a guest…
Six teams named EIT Health Wild Card 2019 finalists

After the Hackathon, the teams will be…
Introducing the 2019 EIT Health Think Tank topic: ‘Optimising Innovation Pathways: Future Proofing for Success’

Join our Digital Town Hall to hear…
EIT Health-backed start-up Starling Surgical wins chance to pitch in US

They join the Medtech Innovator Showcase programme
EIT Health sends five start-ups to pitch at European Life Sciences CEO Forum

Kurt Höller, EIT Health Business Creation Director,…
Freimut Schliess blog post: Digital diabetes and EIT Health

Written by head of CLOSE Innovation Project
EIT Health’s Matchmaking event 2019 features record number of participants and meetings

Finding partners for 2020 projects
EIT Health`s CLOSE Project recognized by researchers from the National Institutes of Health (NIH)
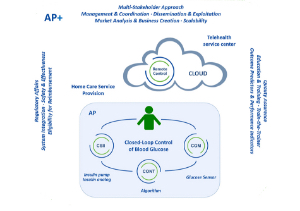
Approach to artificial pancreas called 'very ambitious…
EIT Health Round Table features heads of universities and university hospitals

High-level discussion about synergies and strategy
Meet the UK-Ireland Entrepreneurs: Kaido grows with EIT Health Headstart funding

A wellness platform
Video: Workshop on EIT Health Innovation Project that helps fight superbugs

BL-DetectTool quickly identifies presence of antimicrobial-resistant bacteria
EIT Health adds four Partners as seven other Partners upgrade to Core status

Effective 1 January
Find out about Wild Card in a live webinar, Tuesday at 12:30 CET

Learn how to be part of this…
Start-ups backed by EIT Health Spain garner strong Horizon 2020 support

Five of 17 Spanish start-ups chosen for…
Altran and Sanofi support Wild Card challenge regarding mental and brain health

French Partners get behind 2019 challenges
Video interview: Accelerator’s GoGlobal Medtech activity

Olof Berglund GoGlobal Medtech project leader
Governments get behind EIT Health-backed Stockholm3 prostate cancer test

The Stockholm3 test will become standard in…
The winner of the Sorbonne University Innovation Day has been chosen

Students with an entrepreneurial spirit searched for…
Video interview: JPAST helps with early diagnosis of joint afflictions

Maryam Poorafshar of Thermo Fischer Scientific, the…
Mental and brain health, and digital biomarkers, to be Wild Card challenges for 2019

Jorge Fernandez Garcia, Director of Innovation, announces…
EIT Health Think Tank releases report on Big Data and healthcare at Summit

Download the report
EIT Health Scandinavia names Erik Forsberg as new Managing Director

Interim Managing Director Henrik Cyrén will revert…
Deloitte study ranks German EIT Health partner locations among top tech hubs
Munich leads, followed by the home bases…
EIT Health Digital Town Hall: Join an online conversation about the potential of Big Data in healthcare
See details below to participate in our…
Final KTI 2018 Workshop organised in Erlangen, Germany

RIS Hubs & EIT Health InnoStars promote…
Smart Ageing Camp at the Gdańsk Science and Technology Park

Smart Ageing Camp within the Regional Innovation…
Workshops and networking events organised by RIS Hubs

Consorzio ARCA & Medical University of Gdańsk…
“Berrito” – the winner of the “3 Day Start-up” programme

The programme allowed participants to better focus…
Hernâni Zão’s story: StarShip opened up a new door of opportunities

Hernâni Zão was selected to join the…
Private healthcare and innovations Congress by “Portfolio”

The discussion about healthcare in Hungary involved…
Meet the UK-Ireland Entrepreneurs: Loci Orthopaedics

Bringing essential advances in surgical intervention for…
EmergencyEye showing at PMRExpo, a major communications fair in Cologne
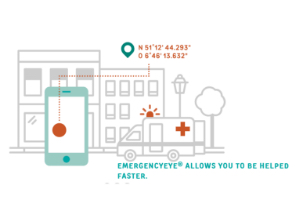
Remote assistance product of EIT Health Innovation…
First EIT Wild Card winner, DX-Labtrack, receiving €2 million to develop real-time sample monitoring

Technical University of Munich, Germany, provides incubator…
FabLab Escape: The game to discover prototyping tools

Students-entrepreneurs play the game and learn how…
Meet Lizanne Svane and her company Tubus Technology

A low-tech device with a high impact…
Sparking ideas for healthcare solutions in areas of ‘Bringing Care Home’ and ‘Real-world Data’

More than 130 participants develop project ideas…
EIT Health Germany announces 2nd Batch of Headstart Awardees 2018

Four additional German and Swiss Start-Ups bound…
EIT Health 2018 HeadStart Winner Inovus Medical announces launch of Bozzini™

An affordable turnkey hysteroscopy simulator
Linxens joins EIT Health France as Network Partner

A Partner designing and manufacturing microconnectors and…
CERITD joins EIT Health France as Network Partner

An innovative clinical research centre in the…
The French start-ups selected for the European Health Catapult Finals

Three French start-ups pass the semi-finals and…
Porto4Ageing: Improving medical plan adherence and the management of Polypharmacy

Non-adherence to medical plans by seniors is…
ICTskills4All: A new project led by an RIS Hub

New Porto4Ageing Reference Site project dedicated to…
EIT Health Smart Ageing Camp at Medical University Semmelweis

Active Ageing can make a real difference,…
InnoStars supports Partners in Innovation Project proposals

Online consultations with InnoStars Managers can be…
EIT Health and EIT Food organise workshop on food and health

Co-creating new strategies to help citizens and…
Smart-Up Lab: 15 ideas and €10K for the best projects

The idea of deep learning AI tools…
Medical University of Gdańsk promotes KTI and EIT Health

E-Health Forum Digital Transformation in Healthcare with…
WE Health Module 3: Empowering Women Leadership in Health Innovation

A three-day workshop in Barcelona supporting female…
Alzheimer’s Challenge: Public can co-create solutions to patient behaviour online

Register now: The collaborative innovation journey begins…
European Health Catapult Biotech Semifinal Winners selected

Seven start-ups will pitch at the EIT…
Finalists Selected to Pitch for EIT Health Germany Headstart Awards Valued at €50 000

Pitching will take place at the EIT…
A recap on the d-HealthyLife pilot training course in Barcelona

This course focused on co-creation methods for…
Video interview: Claire Nassiet on EIT Labelling for education courses

'Those programmes are a bit special. They…
EIT Health’s CLOSE project detailed in scientific journal

Project seeks to enhance artificial pancreas (AP)…
The German start-up Living Brain receives EXIST Grant

Making neurorehabilitation more effective and contemporary
Meet the Innovation Project HealthBed

The unobtrusive monitoring of health-related parameters in…
MOOC: Data Analytics in Health – From Basics to Business
Enroll now to learn more about healthcare…
Impressions on Nordic Life Science Days 2018

An opportunity to network with partners and…
EIT Health launches EU-wide crowdfunding through partnership with aescuvest

Innovative approach to funding innovation
EIT Health MOOC teaches systematised approach to life sciences innovation

Sign up by 1 October 2018
EIT Health partner universities from Germany and Switzerland rated highly in Shanghai Ranking

The Academic Ranking of World Universities has…
EIT Health Germany Partners participate at 7th Annual Symposium ‘House of Pharma & Healthcare’

Enhancing cross-industry collaboration for better health outcomes
Join a Working Group to develop collaborative masters and PhD programmes

EIT Health Campus Degree Programmes activity
Cook2Health’s pilot study presents at the ESPEN Congress

Participating at this Congress triggered many exchanges…
EIT Health community can attend 11-13 Oct. Semmering Symposium In Vienna at special rate

EIT Health CEO and Board Members among…
EIT Health Investor Network open for applications from start-ups

First EU network of investors focused solely…
Interview: A HelloAI Summer School student shares his experience

Guillaume Rollin, a French young physician, participated…
RABBIT programme showcased at European Biobank Week 2018

A programme for implementing innovation to enable…
Vote here for EIT Health’s nominees to the EIT Public Award

To be presented at the 4 October…
Health VentureLab Programme Launch Day

GE Healthcare has introduced the Accelerator programme…
Prof. Dr.-Ing. Joachim Hornegger joins EIT Health Supervisory Board

EIT Health Germany's academic representative
Sameena Conning starting in new position of EIT Health Director of External Affairs

Post created to unite public affairs and…
Activity leader video interview: Jorge Figueira on the StarShip Fellowship

Applying the Stanford Biodesign approach
Third EIT Health Think Tank Round Table hosted by Karolinska Institutet

Better health for European citizens with better…
Gothaer Krankenversicherung is new Partner with EIT Health Germany

A private health insurer
The ‘CLOSE-Lodz-Survey’ with type 2 diabetes patients

The presentation during the 19th Diabetes Poland…
Interview: EIT Health Alumna promotes women’s empowerment in health innovation

Ariadna Diaz, EIT Health Alumna and participant…
Letter on Brexit from EIT Health UK-Ireland Managing Director Leslie Harris

Good news on UK plans for Horizon…
EIT Health participating in the 2018 NHS Health and Care Innovation Expo

Innovative healthtech projects will be showcased at…
EIT Health-backed start-up prepares to launch their Y-shaped toothbrush

FasTeesH receives Headstart funding
Update: Professional Campus and Executive Education Activity Line

Empowering health professionals, health innovators, healthcare providers…
European Health Catapult Finalist SpinDiag showcased on German TV

Fighting drug-resistant bacteria in hospitals
Headstart Awardee OptiMedis joins forces with BKK Werra-Weißner to establish integrated care in Northern Hesse

Fostering an integrated care system in a…
Public invited to 13 August Round Table session on biobanks and registries in Stockholm
An EIT Health Think Tank event
Activity leader video interview: Lefkos Middleton on CARE Campus

EIT Health training initiative seeks to professionalise…
EIT Health Spain names 15 winners of Headstart funding

Support for early-stage start-ups
Go2China: A Training Bootcamp in Groningen

Conducting business in the Chinese medical market
LETI launches its 2nd edition of Medtech Entrepreneur-In-Residence Programme
Leti is targeting segments aligned with medical,…
Project leader writes about P-PALs for Ireland’s frontline

EIT Health Campus activity empowers older adults…
See a video of the first EIT Health Town Hall

Veronika Schweighart, Co-Founder and COO of Climedo,…
Forty-two start-ups chosen for European Health Catapult Semifinals

They will pitch for a chance to…
Healthcare Training @ P3-Stroke

Improving stroke management and cardiac procedures with…
MEDIKURA: A Digital Health start-up from EIT Health Germany’s ecosystem

Improving drug safety with a centralised and…
Tapir-Tapes and Studio Seufz invited to Ottawa International Animation Festival
EmergencyEye will launch a web-series showing how…
Sleepiz: Among EIT Health Germany’s Regional European Health Catapult and Headstart winners

Swiss MedTech start-up develops revolutionary sleep disorder…
Wild Card contestants pitch at end of training and mentoring session

EIT Health will support the best ideas
EIT Health France selects six start-ups for the 2018 European Health Catapult Semifinals

Selected start-ups will have a chance to…
Second EIT Health Think Tank Round Table on Big Data hosted by the University of Oxford

Experts on Big Data in Healthcare suggest…
EIT Health nominates seven innovative people and projects for the EIT Awards

Awards recognise Europe's top innovations and innovators
INnovating the JOY of Eating for Healthy Ageing (INJOY)

EIT Health concludes its ten-day training for…
EIT Health and Nice: At the heart of European health

EIT Health France will support the fight…
EIT Health selects UK-Ireland healthcare start-ups for the 2018 European Health Catapult

Six UK and Ireland Biotech, MedTech and…
EIT Health Innovation Project RAMSES set for first release of EmergencyEye, a potentially life-saving innovation
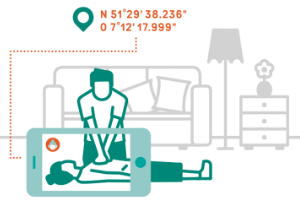
Remote access to smartphone functions in emergency…
New project collaborations emerge at EIT Health UK-Ireland Partner Meeting

Potential projects for the BP2019 2nd Innovation…
EIT Health Scandinavia announces the 2018 European Health Catapult nominations

The nominees will receive training in business…
The French Selection winners of the 2018 Headstart Funding Programme

The eleven start-ups will be able to…
InnoStars selects winners for the Headstart Funding programme

Eight start-ups were selected for the Headstart…
UK-Ireland EIT Health Headstart competition in Dublin: 13 start-ups win €50k each

Chosen from 22 finalists on 17 July…
EIT Health GoGlobal will introduce 13 European companies to the Canadian market

A unique gateway to help firms scale…
After pitches on a ship, EIT Health Germany awards six firms Headstart support

Start-ups get up to €50 000 plus…
EIT Health Spain awards Headstart support to 15 start-ups

Biotech, medtech and digital health innovations backed…
Start-ups to present during EIT Health Start-ups Meet Pharma Demo Day, 26 July

Register to attend the event
‘Hackathon am Ring’ sees hackers attack cardiac arrest

EmergencyEye Hackathon attracted over 100 international hackers…
Some topics chosen for the Bilateral Meeting with EIT Health UK-Ireland, EIT Health Germany

The first set of Round Table topics…
The d-HealthyLife Round Table on co-creation methods

Involving stakeholders in the creation process improves…
Real World Data Workshop in Erlangen

International EIT Health Workshop promotes projects in…
Infographic: 6 Things You Didn’t Know about the UK-Ireland Partner Roadshow

FAQs on BP2019 Call for Proposals &…
Regional EIT Health Highlights for Baden-Württemberg

Report gives an overview of main regional…
EIT Health Education Director Ursula Mühle gives interview on innovative education

Published by SensUs project
Network of Starter Labs maps cooperation

E-Lab partners explore structure and sustainability in…
EIT Health Germany sponsors e-health Forum Start-up Award

evid.one chosen as the winner of the…
The second edition of the EmergencyEye Hackathon

Entrepreneurial enthusiasm to save lives combined with…
Deadline for EIT Community Business Idea Competition now 8 April

Health, food or raw materials ideas sought
Wild Card teams to be chosen 16-20 April in Galway

Winners will receive extensive EIT Health support…
Successful EIT Health Summer School: RAMSES

Inspiring RAMSES Summer School enhancing EmergencyEye to…
Therapy Lens hologram app for dementia patients available for download

Demo for Microsoft HoloLens shows how augmented…
Expert Interview: Co-Creating Innovative Solutions for Health (CRISH)

Expert Maria Piggin tells us more about…
Sensors to prevent Parkinson’s patients from falling

New EIT Health research project launched on…
Dr. Ursula Mühle to give webinar on Campus best practices

EIT Health Director of Campus teaches online…
Gérontopôle de Toulouse is becoming a Network Partner of EIT Health France

Prevention of disability and innovation in the…
Université de Technologie de Compiègne is becoming a Network Partner of EIT Health France

UTC is strongly involved in developing health…
ExMateria is becoming a Network Partner of EIT Health France

ExMateria is an intellectual property law firm…
CRAASH Barcelona launched with a two-day intensive training and final discussion panel

Smart glasses, low cost exoskeletons and anti-stress…
Leslie Harris appointed as Managing Director of EIT Health UK-Ireland

Effective 30 April 2018
EIT Health-supported Premedit set to launch new version of Running Care app

French start-up's e-health solution dedicated to running
Ten start-ups backed by EIT Health present innovative solutions for MT Connect

1,580 trade visitors at twin medtech events…
Wild Card: 12 finalists to pitch healthcare innovations after wild week in Galway

AT THE EDGE tournament identifies transformative ideas…
EIT Cross-KIC Spain presents model for European competitiveness

Spanish EIT KICs present their strategy to…
Three EIT Health partners ranked among top five university-based incubators

UBI Global issues bi-annual awards
WE Health offering training to empower women in health innovation in 2018

Three modules planned for this year
Baxter: New Network Partner of EIT Health France

Baxter brings new competencies to enhance the…
Apply by 15 March for Innovation Game, gamification-based summer school

Course held in 6-17 August 2018 in…
Peptomyc wins the EmprendedorXXI Award

CaixaBank recognises Peptomyc and Bound 4 Blue…
StarShip Innovation Fellowship programme accepting applications until 16 March

Stanford Biodesign Concept and other approaches employed
Expert Interview: Patients as active decision-makers

Q&A with Ania Henley, Patient and Public…
URGO Mentorship Program: First edition

French Network Partner, URGO Group, picks Irish…
Pollar: A success led by Bull-Atos, EIT Health France Partner

The impact of air POLLution on Asthma…
After months of preparation, healthcare start-ups pitch in EIT Health Wild Card 2019 final

Two teams will win €2 million, lab…
CLOSE Consortium meets to map out 2018

Project group defines future activities in historic…
EIT Health UK-Ireland Partners involved in 15 Campus activities

Imperial College London is particularly active
EIT Health UK-Ireland Partners involved in four Innovation Projects

Partners Join 2018 Innovation Projects
EIT Health Spain welcome BIST and Ferrer as new partners

Collaborating with new partners in science for…
EIT Cross-KIC Spain Participates in the Transfiere 2018 Forum

EIT Communities share business opportunities and entrepreneurship…
Accelerator Calls: Apply now for activities assisting start-ups and SMEs

Online application interface is open
The UK-Ireland Partner Roadshow Gathered over 100 Participants

Upcoming important dates and UKI regional innovation…
Smarter Time: The Mobile Artificial Intelligence

Intelligent mobile application helps users achieve a…
Call opens for EIT Health Hubs in Regional Innovation Scheme (RIS) countries

Call runs through 2 March 2018
MOOC promoting Salsa for healthy ageing starts 5 March

EIT Health supports courses, offered in both…
CCentre in 2017: Four courses on citizen-centred ageing and three videos released

More than 100 executives and professionals trained
Meet the EIT Health UK-Ireland Entrepreneurs: Damibu, Headstart/Proof-of-Concept Winner 2016

Damibu’s CATCH app hailed as 'nationally-important' innovation
A 3D multispectral camera for skin scanning

This young start-up designs and produces innovative…
EIT Health BioEntrepreneur Bootcamp – apply now

Online application is open now until April…
EIT opens call for urban mobility and manufacturing Innovation Communities

Competition runs until July 2018
Meet the UK-Ireland Entrepreneurs: Selio Medical, Headstart/Proof-of-Concept Winner 2017
Preventing Pneumothorax and Creating Jobs in Ireland’s…
Affichem wins Special Public Prize in ‘Health Catapult 2017’

Company develops AF243 compound for patients with…
Strategic effort drives Campus Call for Innovative Education activities in 2018

Proposals due by 8 March
Campus accepting proposals for Innovative Education Projects

Application deadline 8 March 2018
Interview: CLOSE crosses EIT Health pillars to ‘close the loop’ for diabetes patients

EIT Health Innovation Project also spawns Campus…
EIT Health nominates outstanding healthcare innovators for prestigious EIT Awards 2019

Recognizing Europe’s successful start-ups, innovative projects and…
UK-Ireland based Innovation Agency and Oxford AHSN’s status upgraded to core partner

Effective 1 January 2018
EIT KICs meet innovators in Lisbon, Kaunas

EIT Health joins dialogues with local innovation…
Dr. Katharina Ladewig appointed as Managing Director of EIT Health Germany

Effective 1 January 2018
Health System Performance and Innovation Meeting

Round tables address possibilities for innovative improvements…
EIT Health Spain holds its 2nd Annual Meeting at the CaixaForum in Madrid

Impressive evolution of the consortium is noted
UK-based Oxford Endovascular Awarded Third Prize in EIT Health European Health Catapult (MedTech)
The UK-Ireland CLC congratulates Oxford Endovascular on…
EIT Health UK-Ireland Welcomes Home Instead Senior Care as New Associate Partner

Find out more about Home Instead in…
Novel motion capture system supports patients’ rehabilitation

EIT Health-supported project offers a wearable system…
EIT Health UK-Ireland Headstart Finalist PhysioMedics Closes Investment Round
Revolutionary online healthcare platform PhysioWizard raised a…
Best project idea awarded on final pitches during the EIT Health StarShip programme

StarShip, the InnoStars Innovation Fellowship programme itself…
Most promising InnoStars start-ups awarded

80 start-ups applied for 2017 InnoStars Awards…
CATCH by Damibu selected to join the NHS Innovation Accelerator

A great achievement for EIT Health UK-Ireland…
Wild Card Challenges for 2018: AI for patient data and antibiotic resistance

Applications will be open between 10 January…
Meyko begins technical studies to industrialise their product

Meyko accompanies asthmatic children to improve controller…
Affichem, BloodLink and Mowoot win audience vote of European Health Catapult contest

Voting held on the first day of…
Announcement of change in EIT Health leadership for a continuing journey of success

From the EIT Health Supervisory Board Chair
First ISAX’s Smart Ageing Innovation Awards held at Irish House of Lords

The Smart Ageing Innovation Awards aims to…
The Spanish Group of Innovation and Knowledge Communities as the spearhead of innovation and entrepreneurship in Europe

The group is made up of EIT…
Hackathon at Imperial College explores blockchain technologies in healthcare

'Smart Trials' wins EIT Health support
EIT Health France welcomes Institut Mines-Télécom as an Associate Partner

TeraLab, a Big Data Platform for Research…
Smart Teacher kicks off with workshop

Educational activity involves cross-KIC cooperation with EIT…
EIT Health, EIT RawMaterials jointly recognise best business models

Innovators in health and raw materials win…
21 finalists named for European Health Catapult competition at EIT Health Summit

They will compete in London during the…
Success of IBV in Horizon 2020 calls involves five EIT Health partners
Two projects accepted
EIT Health Germany will fund six promising start-ups

EIT Health Germany announces Headstart winners after…
TECNALIA wins the European Innovation Award

EIT Health partner developed a new failure…
Leti Launches Medtech Entrepreneur-in-residence Program
The aim is to create startups with…
Empowering Women Entrepreneurship in Health Innovation – 2017 Activities

WE Health aims at enhancing the participation…
New artificial intelligence companion: Vik Sein from Wefight

Wefight develops a new artificial intelligence during…
Visionary Swedish innovators among the EIT Awards winners

Stockholm3 Test awarded best innovation and Multi-mode…
EIT Health course covers Digitizing Home Based Health Care

Hosted by Center for Digital Technology and…
Post-doctoral fellowship proposal, Compiègne, France

CHRONOS: Evaluation of the Motor Functional Age.…
2017 EIT Health UK-Ireland DPhil/PhD Transition Fellowships Announced

At the final round of pitches of…
UK-Ireland Health Innovation Day draws more than 100

Discussion on generating and funding Ideas in…
Imperial College Business School offers short course on ‘Making Accountable Care Happen’

The Imperial College business school, an EIT…
EIT Health-backed cancer therapy firm Peptomyc receives €4.2 million in financing

VC fund Alta Life Sciences Spain I…
Campus – Come and attend this exciting vitality festival for citizens, professionals and researchers

Come and attend this exciting vitality festival…
Women Entrepreneurship in Health Innovation – Capacity Building Module at IESE Business School

WE Health - Final Capacity Building Module…
Neuro-Wear announces production of wearable neural stimulators

EIT Health Innovation Project
Activity leaders in video interview: Living Lab Network

Prof. Maria Teresa Arredondo and Cecilia Vera,…
Going AP+: Creating real patient value with an artificial pancreas

A key milestone in challenging and rethinking…
Seven win UK-Ireland HeadStart/Proof-of-Concept Awards

Event at Oxford coincides with 19 July…
Dynseo to hold senior Olympiad event this summer

Winner of the European Business Plan 2016
Successful completion of InnoDiaCare – the EIT Health Summer School on Digital Innovation in Diabetes Care

Campus project developed in partnership with CLOSE…
EIT Health France welcomes URGO Group in its network

New partner is currently running a Mentorship…
CaixaImpulse program names projects it will support

Some 23 biomedical research projects to receive…
The future of Silver Tech: A challenge for innovation

Exploring ideas, solutions and projects that positively…
Nine start-ups win pitch finals for InnoStars HS/PoC grants

€330 000 in grants given to EIT…
ACS Biotech is seeking additional funds to launch its innovation
Winner of the French CLC’s 2016 Business…
Teams develop and pitch innovations at {Life Science} meets IT Hackathon

International interdisciplinary crews have a weekend to…
EIPP matches investors with EU projects, co-organises EBAN Congress
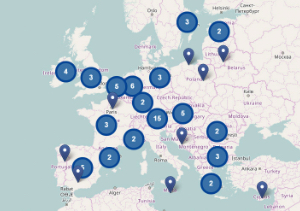
Portal currently lists 45 health-sector investments
Projects to be chosen for Innovation Journey

A one-year supporting program to speed up…
Seven medtech start-ups selected to compete in European Health Catapult finals

EIT Health Spain has announced the winning…
Ten European Companies selected for EIT Health GoGlobal Israel-Canada Programme

Close collaborations will be developed with Toronto,…
EIT MSc in Innovation and Health Care (IHC) celebrates its first graduating class

The Copenhagen Business School celebrated the first…
Campus Call for Summer Schools 2018 now open

EIT Health Campus is now accepting applications…
Partnership with Amgen set to advance value-based innovation

EIT Health and Amgen officially sign partnership…
Kontigo Care reaching the international market

The Swedish e-health company will support European…
Entrepreneurial enthusiasm combined with corporate and academic excellence

More than 75 participants joined German EIT…
EIT Health Campus promotes healthy living at Uppsala run

Students meet patients, public enjoys health screenings
E-lab network workshop on 3D printing

Hands-on 3D printing workshop presented by Swedish…
Trinity College Dublin’s ProACT project partner nominated for a prestigious EU Innovation Radar Award

ProACT will be the first cloud-based, digital…
Fueling the Start-ups of the EU!

Scandinavian EIT Health Alumni start-ups preparing for…
UK-Ireland Partner NUI Galway launches new BioExcel Accelerator

BioExcel Accelerator programme at NUI Galway is…
EIT Health, U. of Copenhagen and UN challenge students to address migrating women’s health needs

The United Nations Population Fund, EIT Health…
The third Joint Business Ideas Bootcamp held in Warsaw

Participants acquired fundamental business planning skills for…
Lili Milani, new EIT Health representative at Estonian partner Tartu University

Research professor in pharmacogenetics at the University…
Event fosters EIT Health Accelerator Pillar in Spain

FIPSE Information Day: instruments for financing R&D&I…
Presenting the 2017 European Health Catapult UK-Ireland Semifinalists: Evolyst (Digital Health)

Award winning evidence-based Health App developer, Evolyst,…
Presenting the 2017 European Health Catapult UK-Ireland Semifinalists: Immersive Rehab (Digital Health)

Interactive physiotherapy programmes in Virtual Reality
Health Integrator: Shifting from reactive care to proactive health

Health Integrator is part of “HealthMovement”, an…
ezyGain conducts research projects in collaboration with hospitals

ezyGain is one of the winners of…
Success of the first French E-lab Summer School on ‘Services marketing for the elderly’

UGA and UPMC co-organised the first EIT…
EIT Health Scandinavia Think Tank: Biobanks and quality registers
The Think Tank brings together groups of…
EIT Health Fellowship Alumni sought to compile strategy report

Alumni of EIT Health Innovation Fellowships are…
Invivox raises €2.8M in bid to be global hub for on-site medical training

Invivox is a global platform, connecting physicians…
Imperial College and Vlerick Business School Boot Camp Hits Second Phase

Selected participants have completed the first week…
Get Help Now: Improving health in the workplace

The consortium consists of a variety of…
University of Copenhagen reports on EIT Health activities

The University of Copenhagen, a core partner…
EmergencyEye® at the CEBIT 2018 in Hannover

Prof. Dr. Guenter Huhle, founder of Corevas…
EIT Health MACH project: An interview with Dr. Carina Benstöm

Children in end-stage heart failures awaiting transplants…
Head of EIT Health-supported start-up wins EU Prize for Women Innovators

No Isolation, a start-up supported by the…
Safety Belt to Go2China: The Chinese health sector market catapult

Safety Belt to Go2China is led by…
Enhancing the contribution of higher education and research to innovation

Cross-KIC event serves RIS regions
HelloAI Summer School: A Summer School on Artificial Intelligence (AI)

Artificial Intelligence might be a useful tool…
Video presents the successful EIT Health Trilateral Meeting
Matchmaking meeting brought together participants from Spain,…
SUN Bioscience – one of EIT Health’s European Health Catapult winners 2017

Together with leading university hospitals in Switzerland,…
EIT Health Germany announces Regional HeadStart Finalists 2018

On 11 July 2018, EIT Health Germany…
Last chance to register: Deadlines extended for Short Courses

Digitalisation to improve hospitals and homecare
Campus eLab generates report on trends in diabetes care

CDTM and EIT Health collaborate in seminar…
EIT Health Germany’s Regional Finalists for the European Health Catapult 2018

The European Health Catapult (EHC) is a…
Starting your Entrepreneurial Journey: WE Health Module 2 supports female founders

During a three-day workshop, participants were given…
UK-Ireland winners announced for EIT Health 2018 Headstart Funding Programme

After two intense days of pitch coaching…
EIT Health-supported projects nominated for EIT Awards 2017

Two start-ups and two Innovation Projects nominated.…
Health VentureLab Weekend workshop in Budapest

The weekend programme consisted of two parts,…
Cross-KIC EIT RIS Business Planning Bootcamp in Tartu/Estonia Successfully Completed

EIT Health, EIT RawMaterials and EIT Food…
EIT Health Alumni win trips to cross-KIC events

Two early registrants going to CONNECT and…
EC platform encourages discussion of Artificial Intelligence policy
Members of the European AI Alliance can…
EIT Health joins Innovation Agency at the International Business Festival

During the event, the Innovation Agency explored…
EIT Health supporting the NHS70 Parliamentary Awards in London

This award seeks to highlight the individuals,…
NHS award supported by EIT Health goes to Community Tissue Viability Services at Pennine Acute NHS Trust in Manchester

"The Person-Centred Care Champion Award" of the…
EIT Health Position Statement lauds EC proposal for innovation support, calls for more funding

EIT Health issued on 28 June 2018…
Ten Start-ups win Innovation Journey finals

Innovative projects will receive grants of up…
Diabetes: A paradigm case for rewarding innovation in value-based healthcare
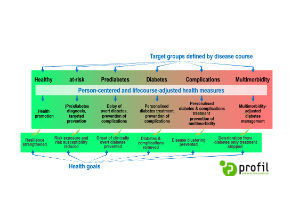
Prof. Dr. Freimut Schliess, Director of Science…
Five Spanish start-ups pitch to win €50k in Headstart funding

A total of 46 proposals were received…
Transition Fellowships deadline extended to June 23

Campus programme targets PhD students The application…
InnoStars selects start-ups for European Health Catapult and HeadStart awards

We are pleased to announce that the…
Towards citizen-centred active ageing and well-being – Phase 2

Innovative training for a society of all…
EIT Health Germany will fund two promising start-ups with innovative emergency and prevention solutions

Two promising projects or start-ups have been…
Activity leader video interview: Joan Escarrabill on involving citizens in healthcare improvement

An explanation of Co-creating Pathways to Promote…
EIT Health France awards 15 start-ups Headstart support for 2019

Meet the exciting new companies receiving €40…
WE Health Module 1: Unlocking your Innovative Potential – first steps towards leadership in Innovation

Do you use the full potential of…
Campus: Innovation Fellowships MOEBIO d·Health Barcelona

Do you want to become a healthcare…
Happytal has started hiring! More than 100 jobs will be created

Happytal opens up offices in new hospitals
Fifteen start-ups chosen during European Health Catapult French Selection

Experts in innovation, business creation and market…
Empowering Women Entrepreneurs in Health Innovation launches registration

The Empowering Women Entrepreneurs in Health Innovation…
Starship – Young talents creating a new era of digital health

Starship fellows met at GE ‘s site…
Potential matches made at German-Spanish-InnoStars Trilateral Meeting

Around 60 participants representing more than 40…
EIT Health Scandinavia names ten winners of Headstart funding

EIT Health Scandinavia names ten winners of…
Cruising and pitching for better health: SHIP 2018

28 start-ups showcase their innovative healthcare solutions…
EIT Health Campus Summer Schools 2017

Explore a potentially career altering experience
Become an Accelerator member and tell us how we can help
Startups join an Accelerator pitch contest at…
Fellows use biodesign to diagnose needs and prescribe solutions

The three types of Campus fellowship programmes…
Activity leader video interview: Alexandre Duclos on E-Labs Corner

Activity leader video interview: Alexandre Duclos on…
Transition fellowships help doctoral candidates

The EIT Health partners universities are collaborating…
{Life Science} meets IT Hackathon in Heidelberg

More than 80 participants from different countries…
Bridgehead chooses 15 more start-ups for support in 2nd selection round for 2019

Incubators, accelerators and clusters provide assistance with…
Digital Town Hall deep dives into EIT Health 2019 Think Tank topic

The 2019 EIT Health Think Tank topic…
Thirty-five EIT Health Success Stories from 2018 approved by EIT

Read about these successes below
RIS Hubs in Croatia, Czech Republic and Latvia join network

They will assist in implementing the EIT…
42 start-ups chosen for the EIT Health European Health Catapult semifinals

The best biotech, medtech and digital health…
Thirteen start-ups win EIT Health Scandinavia Headstart awards for 2019

Winners receive funding to help bring their…
Meet the entrepreneurs heading to the UK-Ireland Headstart Award Finals 2019

22 will pitch for up to €50…
42 start-ups chosen for the EIT Health European Health Catapult semifinals

Following elimination rounds in all of EIT…
Thirteen start-ups win EIT Health Scandinavia Headstart awards

Thirteen start-ups were officially named today as…
EIT Health start-ups earn attention at MedtecLIVE in Nuremberg

19 teams accelerated by EIT Health showcased;…
EIT Health Wild Card 2019 finalists are now in Business Model Validation mentoring phase

Six teams are developing solutions in Brain…
Meet the 16 Finalists for the EIT Health Germany Headstart Awards 2019

Climb aboard! The SHIP – Ship for…
See the video: Digital Town Hall deep dives into EIT Health 2019 Think Tank topic

'Optimising Innovation Pathways: Future Proofing for Success'
EIT Health network takes top spots on Europe’s most innovative universities ranking

Reuters’ 'Top 100: Europe’s Most Innovative Universities'…
EIT Health Bridgehead to support cohort of 16 start-ups in expanding into new European markets

They will be assisted by a network…
EIT Health launches prestigious 2019 Summer School Programme, targeting healthcare leaders of the future

13 courses will run across Europe with…
Go outside the lab: Interview with Oxipit, InnoStars Awards 2018 Winner

Part of a series of interviews with…
Mikołaj Gurdała of EIT Health InnoStars discusses tech and medicine with MedExpress.pl

In the interview translated here, Gurdała highlights…
Jumpstarter, EIT Health Cross-KIC initiative, wins European Association Award

Helping start-ups in cooperation with EIT RawMaterials…
Are you willing to try microgravity in healthcare?

EIT Health has recently been involved in…
New series of interviews with healthcare innovators

EIT Health InnoStars presents inspiring stories of…
EIT Health Partner wins prestigious competition

EIT Health InnoStars' representative will join the…
Video: Innovation Day at Uppsala University

Students immersed in innovative atmosphere
Henk de Jong, Royal Philips: Take Your First Step into Digital Transformation Now

Medical University of Łódź hosted an exclusive…
Spanish Innovation Health sector meets up in Barcelona – see videos

Third EIT Health Spain annual meeting.
EIT Health France reaches out to patients’ associations, citizens’ collectives and others for new collaborations

The EIT Health France team continues to…
MatchPoint: InnoStars joins student initiative to unite young innovators in Budapest

Pilot launched on 6 February at Semmelweis…
Text and videos: Forbes-InnoStars panel identifies healthcare trends

Session held during EIT Health Summit in…
InnoStarter platform helps entrepreneurs find the Accelerator programme that can help them

Developed by InnoStars
EIT Health 2018 StarShip winner garners award in Portugal

EIT Health programme helped start-up receive €50…
InnoStars supports Budapest students in presenting innovations

MatchPoint: A student initiative in Budapest
Glintt hosts other EIT Health partners in European Innovation Networks event

Held in Lisbon in December
Videos: Alumni Board Managers interviewed at the EIT Health Summit

Ayse Tolunay and Ben Hayward talk about…
Students develop innovations at first EIT Health Arrowhead Workshop for Digital European Health Services

Organised through the EIT Health E-Lab Network
Participant details events of 1st EIT Health Alumni Summit in December

Blog post from a Climate KIC Alumnus
EIT Health VentureLab: Start-ups compete with final pitches

GE Healthcare and Hiventures sign an agreement…
Three digital healthcare start-ups awarded in Poland during Innovation Day

A great cooperation of two Polish Medical…
Three of the European Health Catapult 2018 Winners are from InnoStars

Promising start-ups who will make a significant…
The EIT Health healthcare innovators community celebrates its annual Summit in Poland

Over two days, over 400 attendees participated…
Europe’s top health start-ups in Biotech, Medtech and Digital Health win cash and recognition at EIT Health Summit

Finals held for European Health Catapult and…
Wild Card winner Abtrace receives €2 million in EIT Health funding to develop weapon against antimicrobial resistance

Instituto Pedro Nunes, Coimbra, Portugal, to act…
EIT Health Summit 2018: Register by 21 November for our seminal event in healthcare innovation

Join us 4-5 December in Lodz, Poland,…
Students can innovate at I-Day events around Europe in November

Students work on healthcare innovation in teams;…
Six start-ups to vie for €60 000 in InnoStars Awards grants

Competition culminates 16 November 2018 in Budapest
EIT Health Campus announces five selected activities following call for Innovative Education 2018

Innovative methods to teach about innovation
After Stockholm workshop for women, WE Health takes applicants for Munich until 27 May

Programme seeks to help women become successful…
Activity leader video interview: Lisa Walter on Medtech Bootcamp

EIT Health project supports promising start-ups
EIT events changed the lives of more than 200 innovative teams in 2018

€200 000 awarded and more to come…
RIS Hub in Romania promotes EIT Health and innovation in healthcare

Raising awareness of innovation in healthcare in…
Innovation in sports injury prevention wins EIT Health Alumni Showcase pitching

Finals held at Sorbonne University
University of Porto looking for Partners for Living Lab

Porto Living Lab intends to scout innovative…
2018: A year of empowering women at WE Health

Enhancing the participation of women in health…
EIT Health Awareness Raising Event organised by RIS Hub

RIS Hub increasing awareness of EIT Health…
French start-ups awarded by EIT Health during European Summit 2018

Three out of nine winners of the…
EIT Health France announces winning start-ups of Headstart Funding Programme 2018

The winning start-ups received an additional €10…
The winners of the University of Grenoble Alpes Innovation Day have been chosen

Grenoble iDays winners continue their adventure in…
Video interview: Health Movement incentivises healthy behaviour

Leaders of EIT Health Innovation Project explain…
Applicants sought for Wild Card 2019 projects addressing mental and brain health, and digital biomarkers

Apply by 10 February 2019 for the…
i-Days in Poland, Portugal, Hungary and Italy

EIT Health InnoStars Partners joined the Innovation…
Video interview: EIT Health’s Fellowship Network supports innovation and sparks spin-offs

Daniel Mogefors discusses benefits of the Fellowship…
GE5 announced winner of the StarShip 2018 programme

An excellent need-driven innovation tool for athletes…
Networking Session organised for finalists of HINTT Prize 2018

Inspiring networking organised by EIT Health InnoStars…
Praça Vida+ wins award at the VI Regional Congress for Active and Healthy Ageing

Praça Vida+ announced as the winner of…
The Joint Grand Final awards €200 000 in prizes to finalists

40 finalists and 22 top winners shared…
Interviews: Alumni Networking@Frontiers Health Conference 2018, where health meets innovation

EIT Health Alumni share their experiences
Meet the Swedish e-Health company Pilloxa

Product launched and funding round secured in…
Q&A: Abtrace Wild Card project employs AI to fight drug-resistant ‘superbugs’
'Microbes are pretty clever and have evolved…
Big interest in the Run4Health initiative at the Culture Night in Copenhagen

The student driven activity to increase public…
SensArs Neuroprosthetics – The Future of Nerve Stimulation
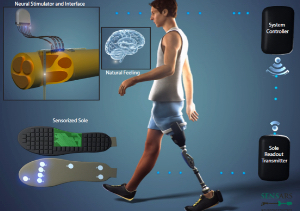
SensArs' first product SENSY allows amputees to…
German Cancer Research Centre fosters patient empowerment

Integrate the patient’s point of view in…
Healthy Life – Healthy Workplace

Ideation workshop in Rotterdam focused improving health…
Three Campus master’s programmes celebrate first graduating class

EIT-Labelled degree programmes hand out diplomas at…
EIT Health Germany Investors Network holds first Round Table in Berlin

Start-ups accelerated by EIT Health Germany present…
The start-up nation: Workshops at Technion in Haifa

Israel Institute of Technology gave the EIT…
A workshop for students on EIT Health MTiH

Discovering the opportunities of the Master of…
The “Kaunas case” and Santaka Valley: The new Silicon Valley of the Baltics
Regional hubs are the main catalysts of…
Joint EIT Health Hub – GoGlobal Programme Survey
Sharing knowledge, best practices, and utilising the…
EIT awards label to RECIPE PhD school to fight antimicrobial resistance
School provides doctoral students with a cross-disciplinary…
Strengthening awareness of EIT Health among healthcare professionals in InnoStars

Smart Technology Mix at HUNGAROMED
European Health Catapult Digital Health Semifinal Winners selected

Seven start-ups will pitch at the EIT…
GENiE: EIT Health hosts 18 Oct. conference promoting innovative education

Copenhagen Business School will play host for…
Mentoring & Coaching Network launches: Start-ups can find expert help they need

Deep well of knowledge from EIT Health…
Start-ups supported by EIT Health Germany progress to European Health Catapult Final Competition

After winning regional pre-selection, Medical Magnesium and…
New advance for the ‘Electronic Health Record’ in Germany
EIT Health Germany Associate Partner Gothaer Krankenversicherung…
EIT Health partners with 1Mby1M virtual accelerator to train more than 100 entrepreneurs

Announcement made at European Health Catapult semifinals
EIT Health UK-Ireland 2018 DPhil/PhD Transition Fellowships Awards Announced

The University of Oxford awarded four PhD/DPhil…
European Health Catapult Medtech Semifinal Winners selected

Eight start-ups will pitch at the EIT…
An interview with Julie Rachline: My experience in the Women Entrepreneur network

Julie Rachline, a French entrepreneur, participated in…
Business in Biobanking and Population Health Data Event: Register by 5 September

EIT Health partners will host the 2nd…
Business plan contest: See what a biobank can do for your start-up and win a €10 000 voucher

EIT Health’s Population Intelligence Lab offers support
Caixampulse will help 20 biomedical projects towards the market

The 4th call of CaixaImpulse programme guides…
Assessing regional innovation ecosystems in the InnoStars regions

Using the Action Plan Finder tool developed…
University of Porto’s technology will help improve spermatozoa quality

Improving the way sperm is preserved for…
The University of Porto meets UC Berkeley

'Leadership Week' in Porto focuses on entrepreneurship…
Citizen Toolkit testing at 3 Days of Health event

Medical innovation created in the regional community
Health VentureLab: Powered by GE Healthcare

Explore and develop health-related business ideas
Competition and coaching programme for Life Sciences businesses

Experienced mentors and coaches help in getting…
Welcoming EIT Health’s newest partners: kENUP Foundation and CRU
Partners in healthcare and innovtion
Hub4AIM: Hub for Accelerating your Innovation in Medtech

Support early stage innovative medtech project in…
InnoStars start-ups selected for the Semi-Finals of the 2018 European Health Catapult

EIT Health has chosen the six best…
The InnoStars Awards training programme has started

EIT Health InnoStars Awards' top 25 start-ups…
Train the Healthcare Manager Trainer programme 1st edition: Register by 31 August 2018

This programme is addressed to programme directors…
EIT Health and Merck explore innovations at Curious 2018 Conference

Shaping the global future of science and…
InnoStars cooperates with the EURAXESS network

Make full use of the opportunities offered…
EIT Alumni CONNECT 2017: A correspondent reports

From 15-16 October, EIT Alumni CONNECT 2017…
Seven Digital Health Start-ups selected for the European health Catapult Final in London

Digital Health Semifinal organized by both French…
Call for Interest – Lead of the Health and Medical Data Analytics Working Group

EIT Label for Master and Doctoral Programmes
Twenty promising start-ups win €7k to develop business plans
Winners will go on to compete for…
EIT Health InnoStars holds innovation road show

Innovation Open House lets partners host information…
Cross-KIC Business Planning Bootcamps in Tartu and Budapest

Entrepreneur with a strong business idea but…
EIT Health CEO to speak at conference promoting socially responsible research and innovation for health

EIT and EESC will host a 18-19…
LaunchLab page launches, in anticipation of September pressure cooker

A landing page was launched in preparation…
Total News Articles: 724